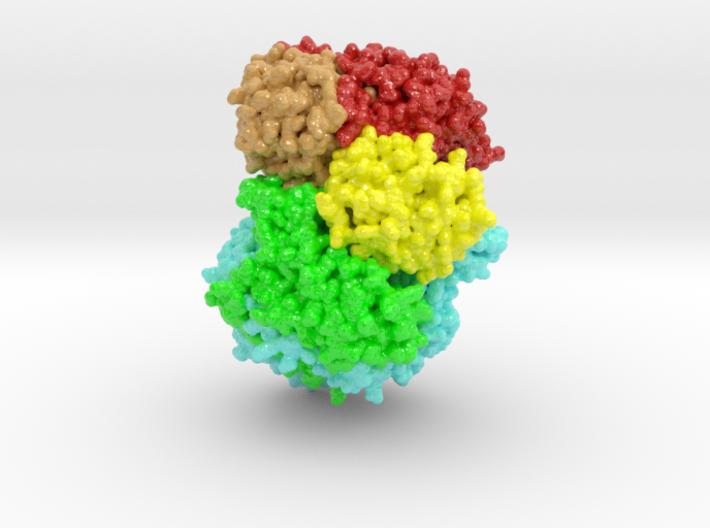
Negative-strand (NS) RNA viruses initiate infection with a unique polymerase complex. This protein complex mediates both mRNA transcription and subsequent genomic RNA replication. Domains for both functional interactions are contained within a single Large polymerase protein (L-protein).
Protein Description 5A22
Large Protein (L-protein) RNA viruses include both Ebola and rabies viruses. We have determined by electron cryomicroscopy the structure of the vesicular stomatitis virus (VSV) L protein. The density map, at a resolution of 3.8 Å, has led to an atomic model for nearly all of the 2109-residue polypeptide chain, which comprises three enzymatic domains (RNA-dependent RNA polymerase [RdRp], polyribonucleotidyl transferase [PRNTase], and methyltransferase) and two structural domains.
Biologic Explorer 5A22
Http iframes are not shown in https pages in many major browsers. Please read this post for details.The RdRp resembles the corresponding enzymatic regions of dsRNA virus polymerases and influenza virus polymerase. A loop from the PRNTase (capping) domain projects into the catalytic site of the RdRp, where it appears to have the role of a priming loop and to couple product elongation to large-scale conformational changes in L.
Model Description
Biologic model of L-protein of Vesicular Stomatitis Virus 5A22 3D printed in Multi-colored Plastic and Full-color Sandstone.