Deadly to newborn babies, the Human Respiratory Syncytial Virus (HSV) is a major cause of lower respiratory tract infection. Dynamic structural changes in the Fusion F Glycoprotein reveal and hide binding sites, making targets of new therapies difficult to identify. Explore RSV Fusion F Glycoprotein-3RRR and learn more about its Postfusion 3D structure.
Model Description
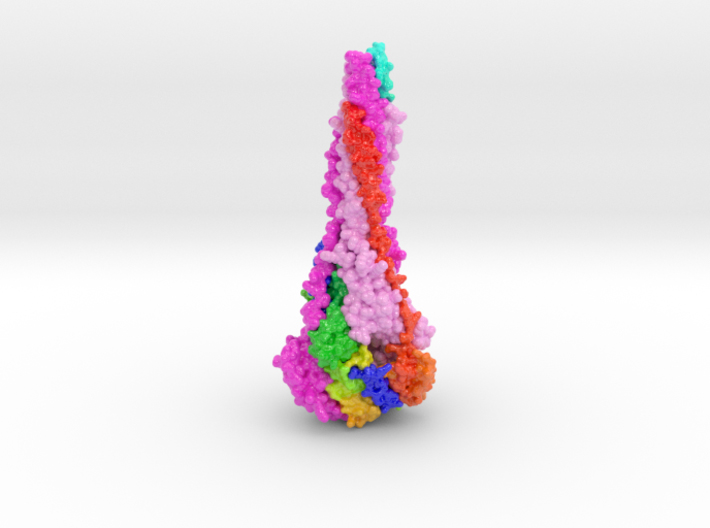
3D printed protein models of RSV Fusion F Glycoprotein visualize the conformational changes of this viral protein during membrane fusion. Each chain is colored uniquely, with one chain colored blue to red from N to C-Terminal.
3D Print RSV Fusion F Glycoprotein-3RRR
Using the interface below, configure the scale and materials using the horizontal slider and drop-down menu.
Protein Description 3RRR
Respiratory syncytial virus (RSV) invades host cells via a type I fusion (F) glycoprotein that undergoes dramatic structural rearrangements during the fusion process. Neutralizing monoclonal antibodies, such as 101F, palivizumab, and motavizumab, target two major antigenic sites on the RSV F glycoprotein. The structures of these sites as peptide complexes with motavizumab and 101F have been previously determined, but a structure for the trimeric RSV F glycoprotein ectodomain has remained elusive. To address this issue, we undertook structural and biophysical studies on stable ectodomain constructs. Here, we present the 2.8-Å crystal structure of the trimeric RSV F ectodomain in its postfusion conformation. The structure revealed that the 101F and motavizumab epitopes are present in the postfusion state and that their conformations are similar to those observed in the antibody-bound peptide structures. Both antibodies bound the postfusion F glycoprotein with high affinity in surface plasmon resonance experiments. Modeling of the antibodies bound to the F glycoprotein predicts that the 101F epitope is larger than the linear peptide and restricted to a single protomer in the trimer, whereas motavizumab likely contacts residues on two protomers, indicating a quaternary epitope. Mechanistically, these results suggest that 101F and motavizumab can bind to multiple conformations of the fusion glycoprotein and can neutralize late in the entry process. The structural preservation of neutralizing epitopes in the postfusion state suggests that this conformation can elicit neutralizing antibodies and serve as a useful vaccine antigen.
RSV Fusion Glycoprotein Realtime-3D Biologic Explorer
Use the interactive realtime 3D Biologic Explorer to visualize the internal anatomy and dynamic conformation changes of the RSV Fusion Glycoprotein. (Best viewed in a full-screen desktop browser).
