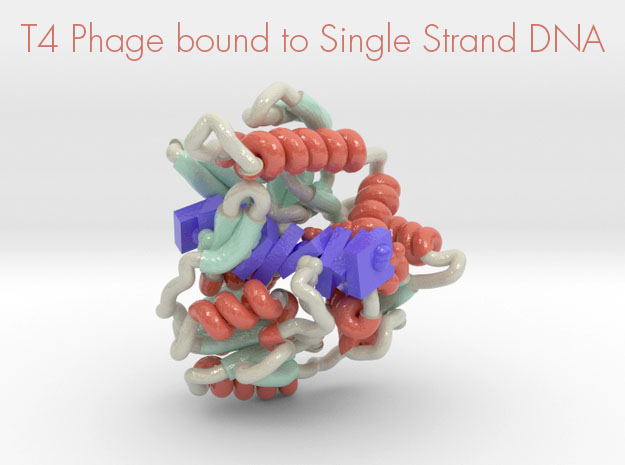
T4 Phage bound to Single-Stranded DNA
3D print of T4 Phage bound to single stranded DNA. Multiple materials and sizes available.
Protein Description 3upu
Helicases move on DNA via an ATP binding and hydrolysis mechanism coordinated by well-characterized helicase motifs. However, the translocation along single-stranded DNA (ssDNA) and the strand separation of double-stranded (dsDNA) may be loosely or tightly coupled. Dda is a phage T4 SF1B helicase with sequence homology to the Pif1 family of helicases that tightly couples translocation to strand separation. The crystal structure of the Dda-ssDNA binary complex reveals a domain referred to as the “pin” that was previously thought to remain static during strand separation. The pin contains a conserved phenylalanine that mediates a transient base-stacking interaction that is absolutely required for separation of dsDNA. The pin is secured at its tip by protein-protein interactions through an extended SH3 domain thereby creating a rigid strut. The conserved interface between the pin and the SH3 domain provides the mechanism for tight coupling of translocation to strand separation.
Biologic Explorer: 3UPU
Http iframes are not shown in https pages in many major browsers. Please read this post for details.Model Description
This 3D printed protein model of T4 Phage bound to Single-Stranded DNA visualized by the protein’s ribbon backbone structure (helices red, sheets teal, coils white) and DNA (purple) as slabs. This model was created from PDB ID: 3upu and printed in full-color.
3D Print T4 Phage bound to Single-Stranded DNA