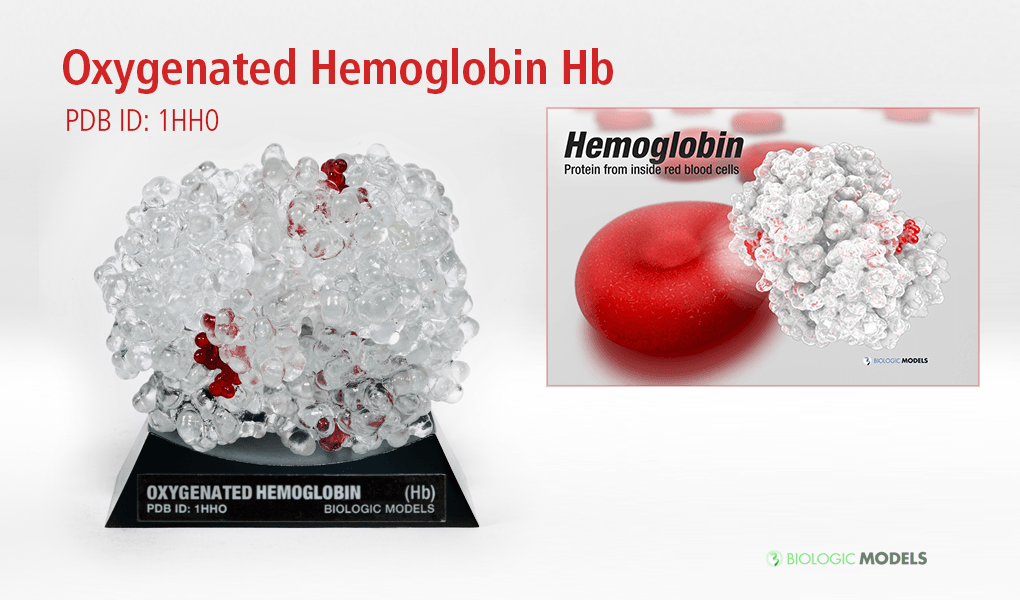
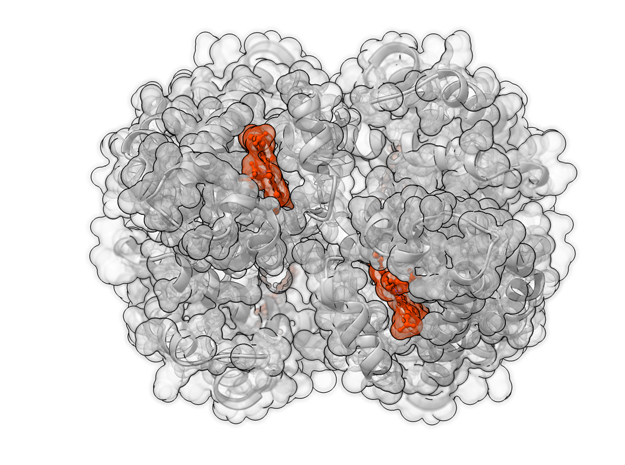
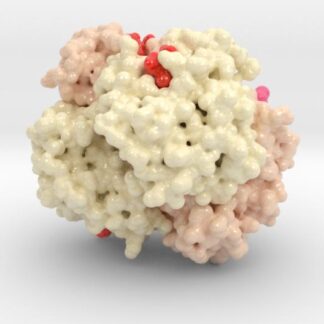
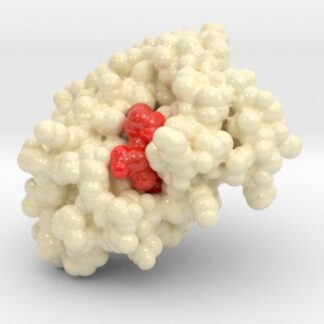
Oxygenated Hemoglobin Hb
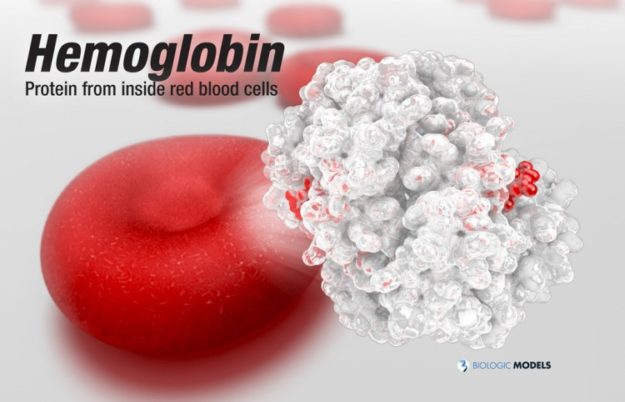
Hemoglobin, the protein from inside red blood cells, transports oxygen molecules throughout the body. Explore its 3D structure with a 3D printed biologic model of Oxygenated Hemoglobin Hb.
Oxygenated Hemoglobin Hb
Hemoglobin, the protein from inside red blood cells, transports oxygen molecules throughout the body. Its macro-molecular symmetrical structure just the first interesting characteristic of this fascinating molecule. Explore its 3D structure with our 3D printed protein models of Oxygenated Hemoglobin Hb.
Protein Description 1HHO

Oxygenated Hemoglobin (Hb) is the protein from inside red blood cells that transporters small molecules like Oxygen (O2) throughout the body. It is a specialized protein exhibiting complex molecular behaviors. Hemoglobin is an important protein to life, specialized at binding and carrying one of the most fundamental molecules for life on our planet, Oxygen. Despite hemoglobin being millions of times smaller than anything we can see with our eyes, we can experience hemgolobin in our everyday life. Hemoglobin gives our blood its red color. When our cheeks become flush with embarrassment, it’s hemoglobin that makes them look rosey. The immediate consequence of taking a breath is for hemoglobin to bind oxygen and change shape. While it may be a tiny, we can draw a deeper understanding of the world around us through this little thing.
Protein Location
Hemoglobin the most abundant protein inside red blood cells. But this isn’t the only place we find Hemoglobin throughout nature. Various forms of Hemoglobin can be found through the body performing alternative functions, like oxygen storage.
Media Gallery
Protein Structure
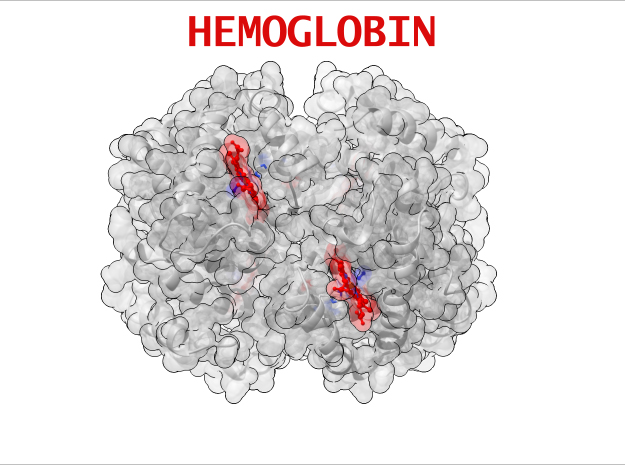
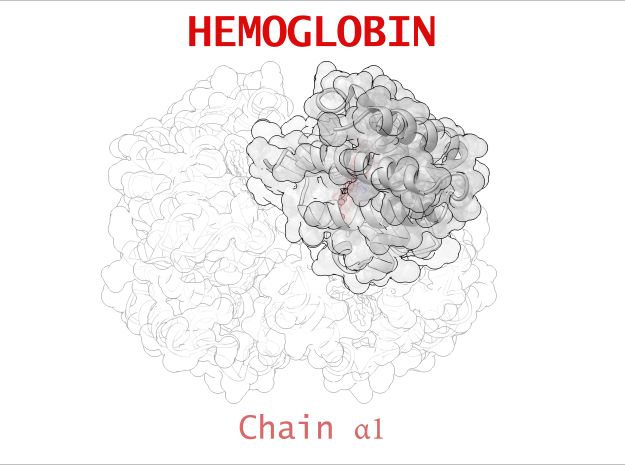
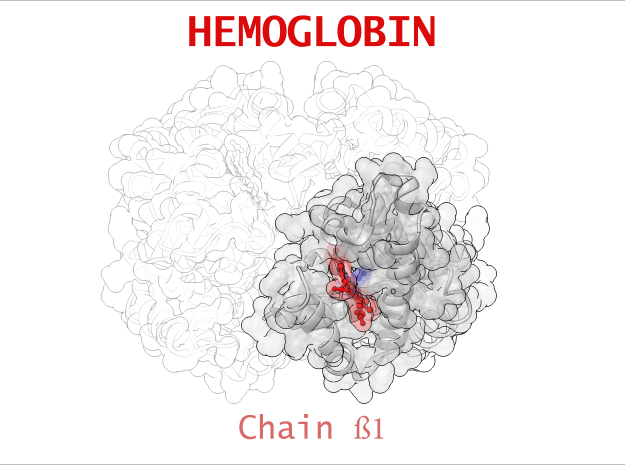
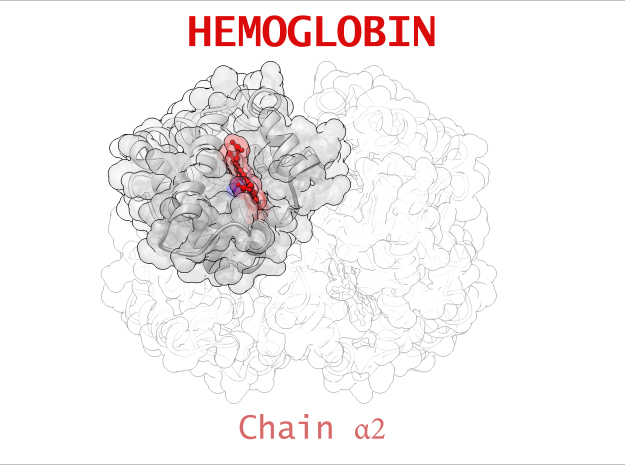
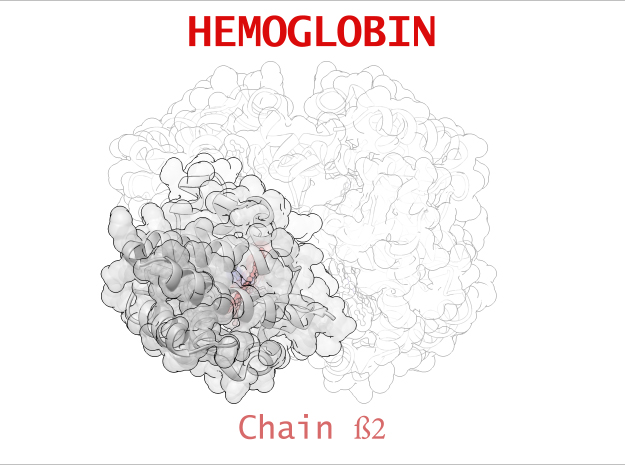
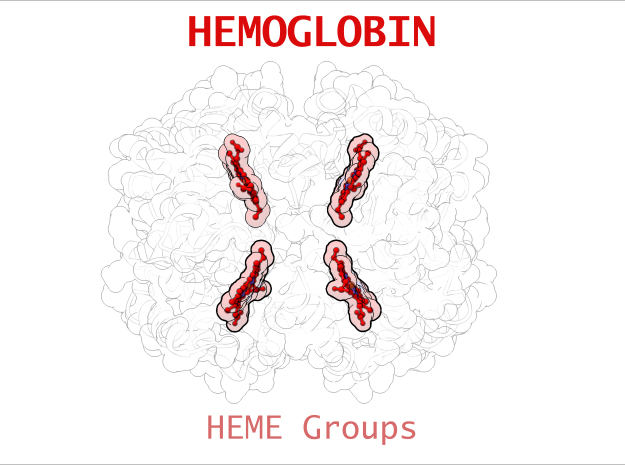
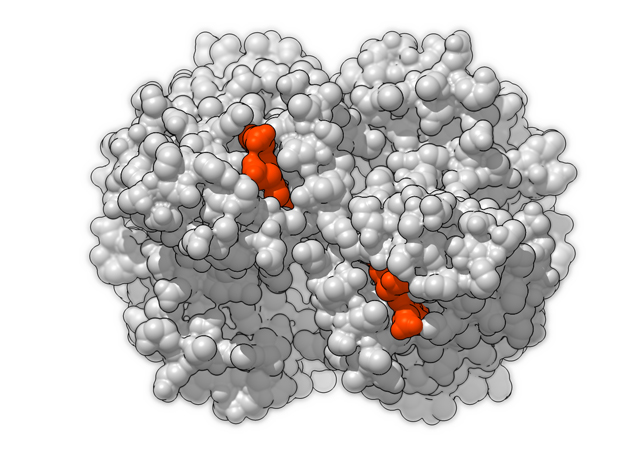
Hemoglobin is a relatively large protein, it’s reported resolution is 2.1 Å. It is composed of four protein chains: 2 Alpha chains and 2 Beta chains. Each chain contains a single ring-like HEME Group (red x 4) with an iron atom core.
3D Animation
Hemoglobin Biologic Model 360 Loop from Biologic Discovery on Vimeo.
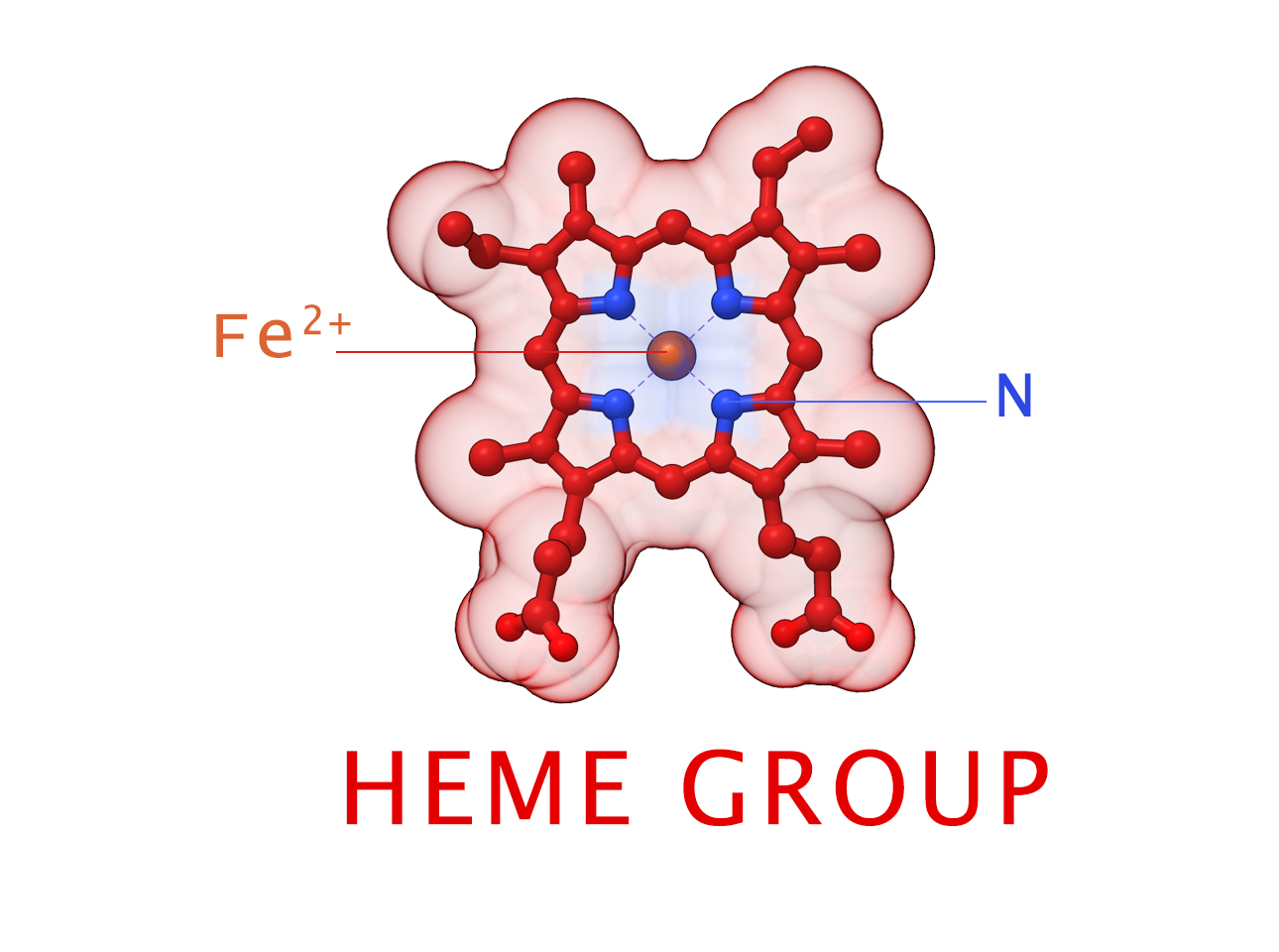
HEME Group
Hemoglobin has an important function to perform in the body and carries around a specialized metal tool to do it. HEME Groups are disk-like rings, one for each globulin chain subunit. The HEME Group is an intriguing molecular structure with genetic origins that scientists believe go all the way back to primordial earth and the original forms of life on the planet.
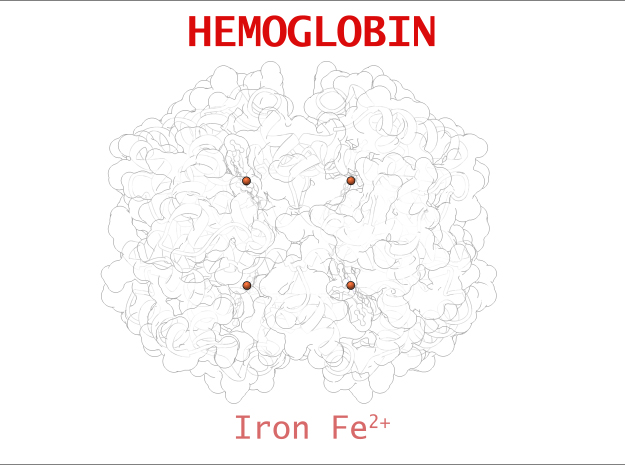
The core of the HEME Group is a porphryn ring, four pyrrolic groups each with Nitrogen atom that bind a metal ion and hold it in place. In the case of HEME Groups, that metal ion is Iron Fe(II). This same ring like configuration is seen in countless proteins throughout nature and used to transfer electrons between compounds and perform a variety of protein functions. For instance, HEME Groups can be found in proteins throughout the brain in synapses and play an important part in creating new memories.
Hemoglobin Anatomy
| Structure | Residues | Atoms |
| Chain α | 141 | 1069 (x 2) |
| Chain ß | 146 | 1123 (x 2) |
| HEME Group | 1 | 43 (x 4) |
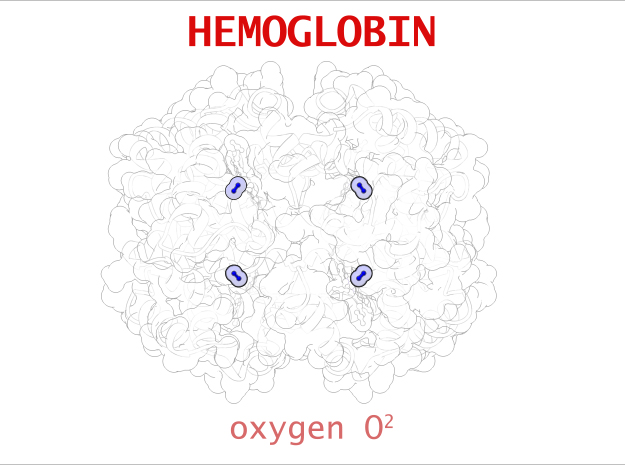
| Oxygen | 1 | 2 (x 4) |
Biologic Explorer: 1HHO
Explore the protein dataset used to create the 3D printed protein model of Oxygenated Hemoglobin Hb 1HHO.
Http iframes are not shown in https pages in many major browsers. Please read this post for details.Protein Function
In vertebrates like you and I, Hemoglobin is responsible for transporting oxygen molecules from our lungs to the cells of our bodies. Hemoglobin’s like a molecular Taxi, 1 hemoglobin can pick up at most 4 Oxygen passengers. It transports Oxygen long distances, from our lungs to the tips of our toes.
Oxygen Transport
Every time you take a breathe, Hemoglobin is hard at work transporting oxygen. Oxygen molecules rush into the lungs as our alveoli expand. Blood vessels line the perimeter of each air sack where hemoglobin inside of red blood cells rushing through the capillaries pick up their molecular cargo. Within moments, those red blood cells have carried oxygen all the way to the tips of your fingers and toes. Along the way, the subtle cellular demand for Oxygen pulls molecules off Hemoglobin 1 by 1 until finally releasing it’s nutrient cargo. Hemoglobin is such an effective transporter of small molecules, it even picks up Carbon Dioxide for its return trip to the lungs.
High-performance Oxygen Storage Device
Hemoglobin is found in more places in the body than just red blood cells. The brain requires a sensitive balance of Oxygen at all times. Since hemoglobin deftly manipulates Oxygen, certain types of neurons will access the Hemoglobin gene in its DNA and synthesize a storage device. It’s believed some neurons operate at a higher metabolic rate than others. For this reason, they have a higher demand for Oxygen.
Leghemoglobin Protein Protector
Would you believe hemoglobin is also a protector of cells? It’s true. Not in humans, or vertebrae though. Legumes. In 1982, Kjeld Marcher and Desh Pal Verma first isolated the genes that encoded for this out of place hemoglobin protein, calling it Leghemoglobins due to the remarkable similarity to mammalian hemoglobin. (IMAGE) It was difficult at first to conceive why a plant would make its own Oxygen transporting protein, since a legume can make its own oxygen using chlorophyll. These scientists began investigating the cellular machinery in the surrounding “nodules” which coded for leghemoglobin to understand more about what a hemoglobin-family protein might be doing there. They speculated that these nodules contained specialized bacteria that convert nitrogen from the environment into a form the plant can use. This sensitive cellular machinery is susceptible to damage from rampant oxygen migrating throughout the cells. Since Leghemoglobin would have a strong Oxygen affinity, Marcher and Verma speculated that the protein scavenged cells for oxygen protecting cellular machinery. After that time, scientists have found hemoglobin proteins in every form of plant studied, even those that form no symbiotic relationship with bacteria. As far as we can tell, nearly all forms of life on the planet currently use HEME-globulin proteins to manage Oxygen molecules inside cells, either for transportation, management or protection. Only something truly ancient could be found in life forms as diverse as bacteria, plants and animals.
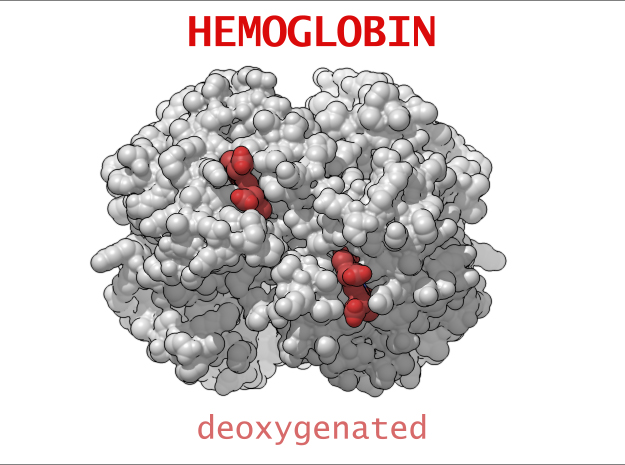
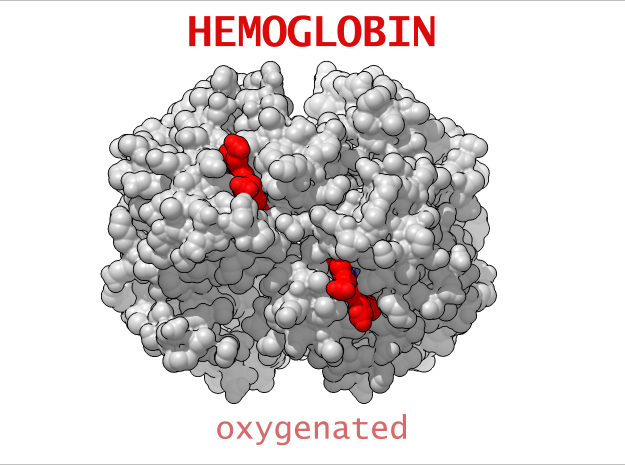
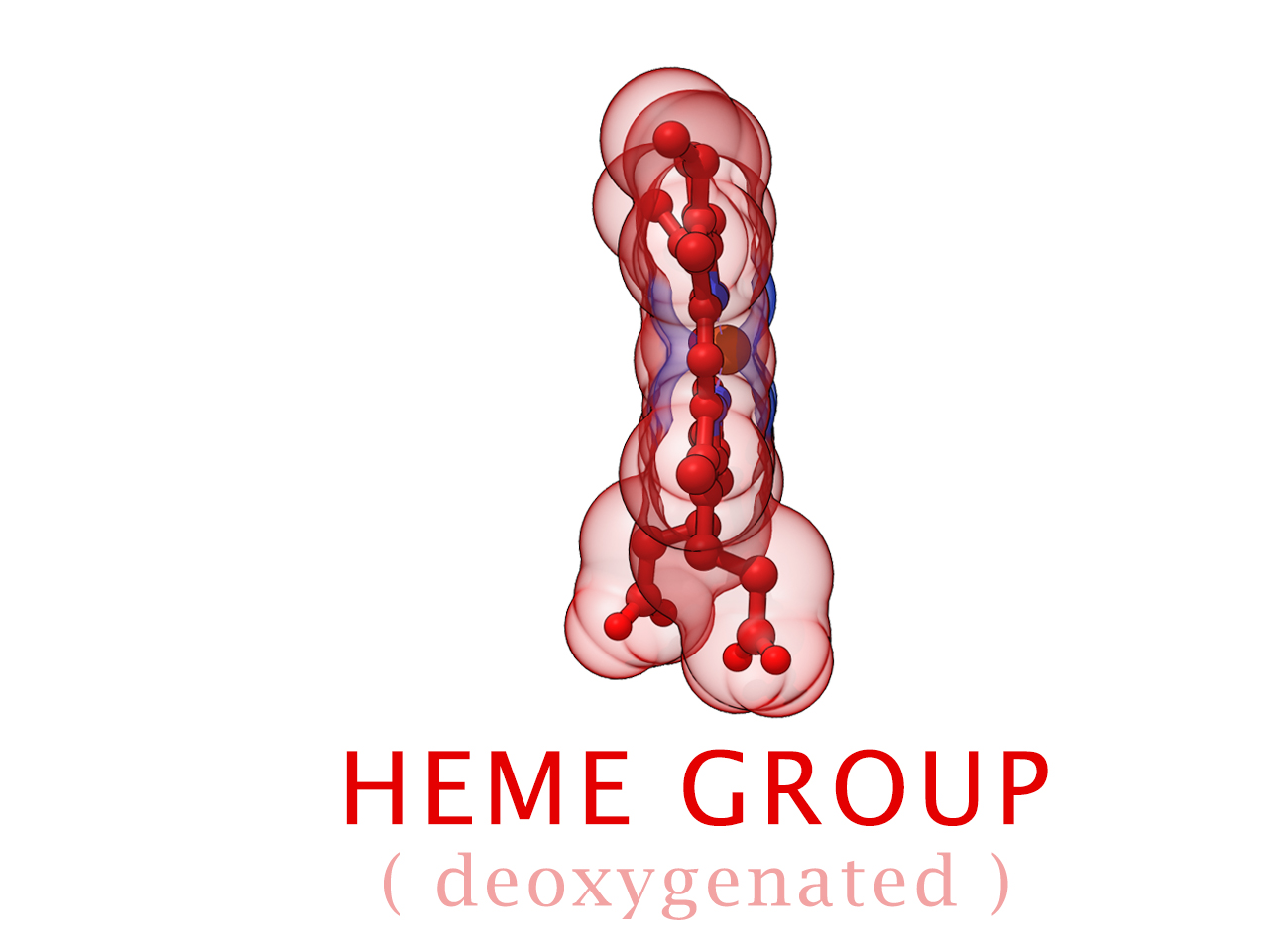
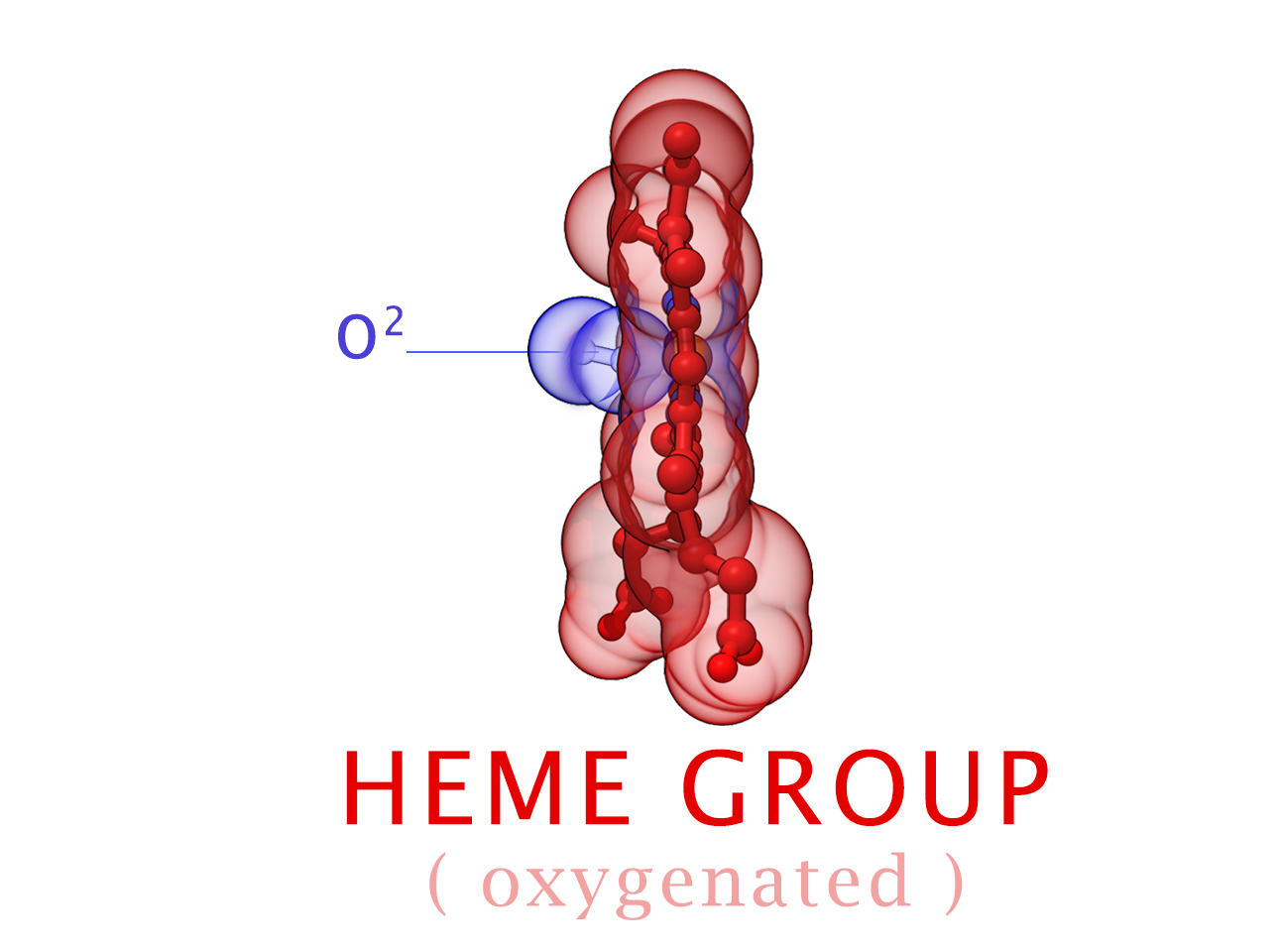
Conformational Changes (R and T States)
The 3-dimensional shape of Hemoglobin is characterized by two specific stable positions referred to as the R and T States (Relaxed and Tense). Oxygenated Hemoglobin is Relaxed and flexible with a high affinity for oxygen. De-oxygenated Hemoglobin is tense, rigid and inflexible with a low affinity for oxygen.Cooperative BindingHemoglobin is a wonderfully complex protein. It’s quaternary molecular structure fine tunes and enhances the individual behavior of a single subunit. One example of this complexity of behavior is something known as “cooperative binding.” Simple put, the more oxygen that binds to a single hemoglobin, the greater hemoglobin’s affinity becomes to bind more oxygen. This interesting adaption is all thanks to the tiny changes in the position of Iron atoms bound to the center of each HEME group.
Role in Health and Disease
Hemoglobin is unique protein. Every single breath, we directly experience with something millions of times smaller than something we can see with our eyes; amazing to think something so small can actually cause us pain. Hemoglobin’s is affected by a number of things; the genes we are born with, the food we eat, the environment around it, and even the air we breathe. Its role in our body is critical to maintaining our health. A number of physiological systems impact the creation and function of Hemoglobin. It is a target for diagnostic measurement and directly connected to a number of different physiological conditions.
Sickle Cell Anemia
Sickle-Cell Anemia is a form of Anemia caused by a genetic mutation in the Beta chain of Hemoglobin. Swapping just one amino acid for another, the mutation causes life-threatening consequences for people suffering from this condition. In its Deoxygenated form (T State), these mutated Beta chains attaches to an adjacent Alpha chain of a neighboring Hemoglobin. One after another, Hemoglobin links to creating a rigid, protein fiber.
Instead of a red blood cell loosely holding a bag of marbles, Hemoglobin fibers deform the shape of the RBC into a rigid Sickle- or C-Shape. In this state, the sickled red blood cell clogs tiny capillaries, digging into and damaging tissue causing cardiovascular problems including pain! The deformed cells are also fragile. They easily rupture causing a loss of Hemoglobin and a decrease in Oxygen transport. Often the body is not able to keep up with the rate of RBC destruction by producing new ones. The disease, while mostly found in tropical regions, results in decreased longevity for those diagnosed with it.
Carbon Monoxide Poisoning
CO is a dangerous gas because of its increased affinity for Iron. CO’s affinity to Iron is 230 times stronger than O2. This means that CO will out-compete Oxygen to bind at the HEME sites, and bind to it stronger, less reversibly than Oxygen. Treatment for CO poisoning involves immediate removal from the affected area. If severe enough, pure Oxygen therapy is necessary to dissociate the CO from Hemoglobin.
Diabetes and the HbA1c Blood Test

Hb vs HbA1c Diabetes Education KitDiabetes has a negative effect on many of the body’s metabolic functions and even affects Hemoglobin. People with diabetes have difficulty removing sugar from their blood stream. If left in circulation too long, glucose will begin to accumulate on proteins, like plaque does to our teeth if we don’t brush regularly.If you’d like to learn more about diabetes, check out our interactive Glycated Hemoglobin HbA1c Teaching Model.
Protein Evolution
Imagine a time long ago, after celestial earth formed and yet nothing more than rock, water and gases. We may never know for sure if life sprang forth naturally from our planet during this time or if instead it was deposited here by meteors. What we do know is that fossil records trace life on our planet back to a time 3.6 billion years ago. Scientists now believe the evolution of Hemoglobin can be traced all the way back to the very earliest forms of life.
Ancestor Protein
Purchase Hemoglobin Models
Purchase one of our custom made acrylic or 3D printed Glycated Hemoglobin HbA1c and Oxygenated Hemoglobin Hb Models.
3D Print Hemoglobin Models
Models available in both Full-color Sandstone and Full-color Plastic.
Interactive Glycated Hemoglobin A1c Model (Acrylic)
This model of Hemoglobin A1c is based on x-ray crystallography data sets: 1HHO, and 3B75. These models are cast by hand in high quality, durable and washable plastics. Model dimensions are 4in x 3cm x 3in. Each model comes with a custom made display base.
3D Print Other Hemoglobin Models
This is a 3D print of Glycated Hemoglobin (HbA1c), created from PDB ID: 3B75. The globulin chains (2x A,B) of this model are colored white, HEME groups red, and Oxygen Molecules Blue. This model also visualizes the HbA1c diabetes blood test by showing how sugar molecules (cyan) attach to proteins changing their shape and decrease its functionality. Based on simulations of potential glycation sites, we have isolated the Lys binding residues (medium Grey) and attached sugar molecules. When compared to our normal Oxygenated Hemoglobin (Hb), you can quickly explain both what the HbA1c blood test examines, as well as why it is so important for patients to control their blood glucose.
Custom 3D Print Request
Request a custom 3D printed protein model. Send us the protein name and PDB ID of the protein you’re interested in printing and we’ll get back to you with a feasibility analysis and estimate for printing.
[ninja_form id=6]