Zaltrap MOA 3D Animation
Depicting dynamic macro-molecular structures is an exciting part of our storytelling process. See some examples of this in our Zaltrap MOA Medical Animation.
Depicting dynamic macromolecular structures is an exciting part of our storytelling process. See some examples of this in our Zaltrap MOA Medical Animation.
Here is an example of some recent work we did for Regeneron and their Zaltrap product line. Below are samples from various stages of our creative process to bring this science narrative to life.
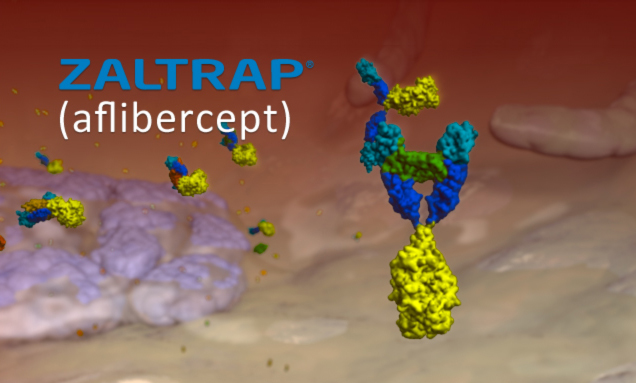
Early on in our creative briefings, it became clear their scientists wanted to communicate the high performance nature of their product. To showcase this performance and sophistication, we harnessed the protein utilized to create their compound and sampled the atomic structures needed to recreate as digital models.
Zaltrap Character Creation
Following early character reviews with the client, including multiple visits to their headquarters to review drawings and models, we quickly progressed to script writing and storyboard finalization. Characters like the one seen here often accompany scripts and storyboards to provide a throughout visual interpretation of the proposed animation.
Zaltrap Protein Assembly

The hero character of this story is a conjugate protein, made up of domains from two different receptors and the IgG1 domain of the IgG Fc antibody.
Storyboards
Storyboards are catered to medical scripts. We work with medical directors and writers to create a narrative structure that allows for viewers to learn about and remember new ideas. Storybaords are a way to go from pencil sketches to refined illustrations that show clients exactly what they’ll see in their final 3D animation.

Med-Legal Approval
As is often required when creating MOA animations for pharmaceutical clients, script, characters, Rough Cut and Final Cut all go through medical-legal review processes to ensure scientific accuracy. We excel at helping our clients navigate the path of review in order to quickly achieve legal approval.