Description
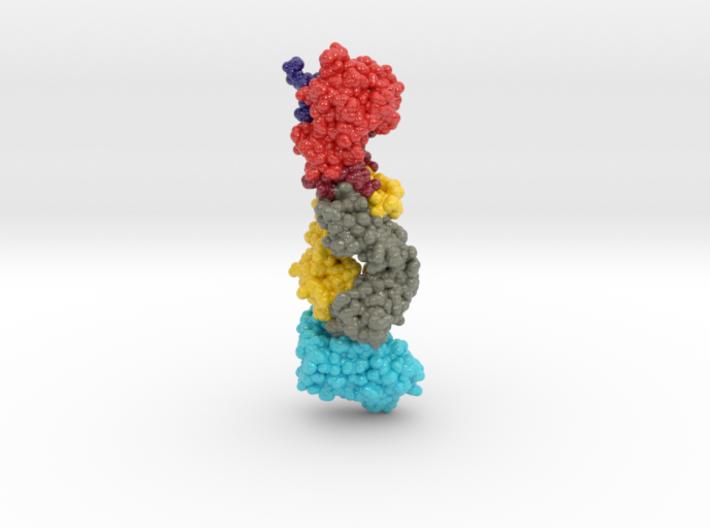
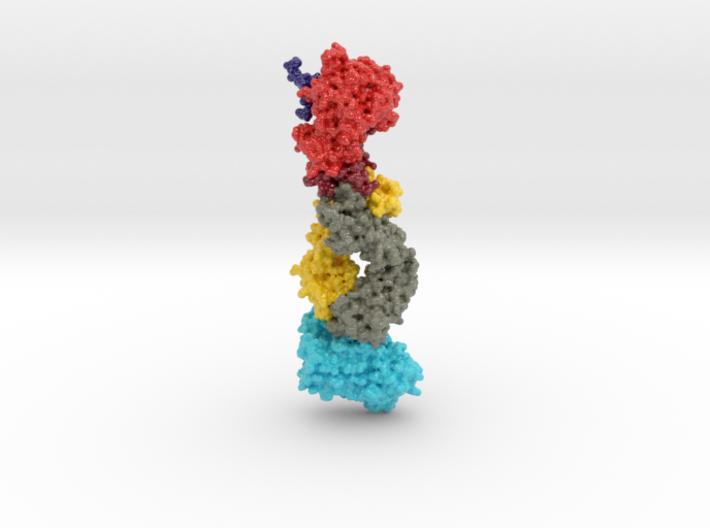
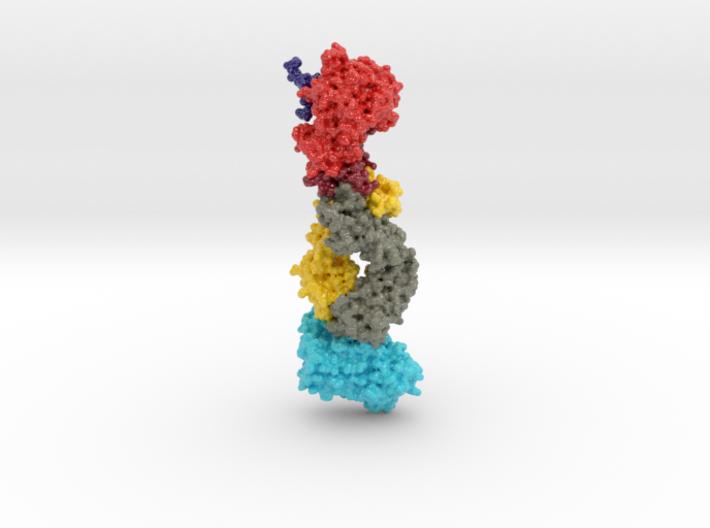
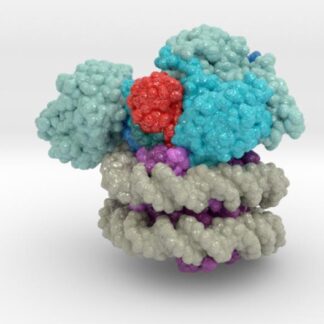
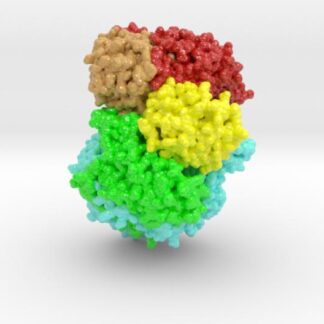
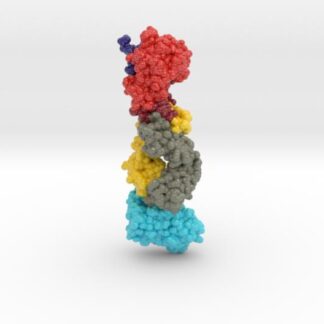
This 3D print of MT-3724 demonstrates a unique mechanism of action of this immunotoxin. MT-3724 is a new type of immunotherapy developed by Molecular Templates.
Protein Description
This cytotoxic drug utilizes their innovative Engineered Toxic Bodies (ETBs) drug delivery platform to trigger cell death. MT-3724 is being developed for the treatment of non-Hodgkin’s Lymphoma and harnesses the immunotherapy power of antibody receptor-specific targeting combined, a bacterial process for cell invasion that infects cancer cells with ribosomal inhibitors that strangle protein synthesis. MT-3724 demonstrates a unique mechanism of action, packing a cytotoxic 1-2-3 cancer killing punch.
For international customers outside the US, please visit the model on our 3D printing service’s international website: LINK
Select the desired material finish and size below. Matte finish applies a UV protective, semi-glossy coating. Natural finish does not.